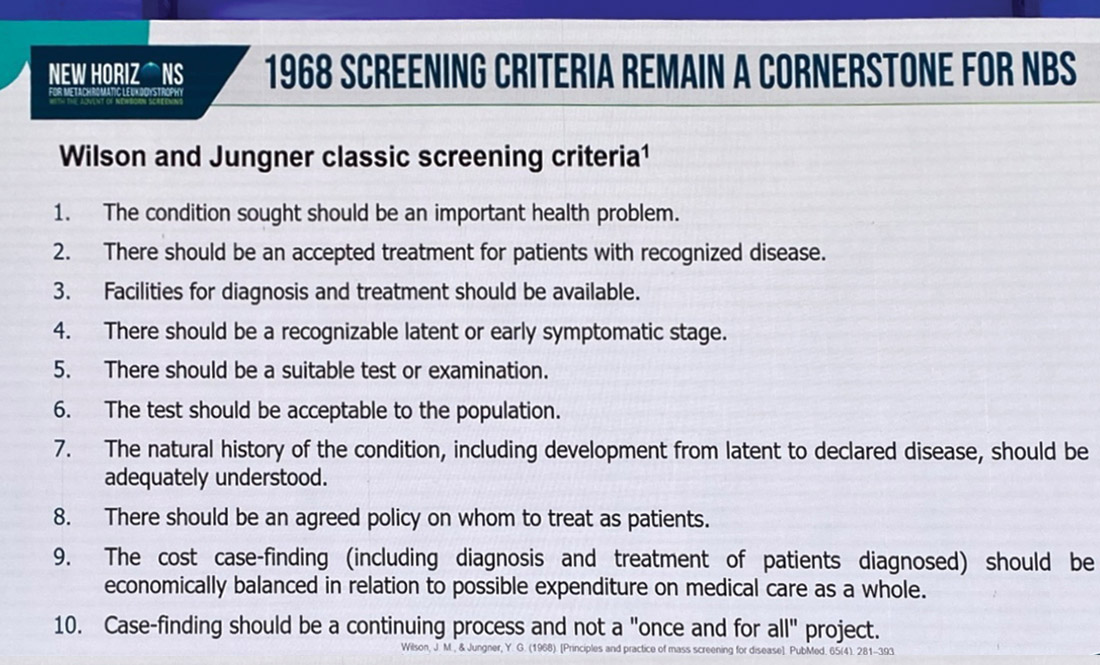
We are delighted to announce that Atidarsagene autotemcel (Libmeldy®) has been recommended as an option by NICE (The National Institute for Health and Care Excellence) for treating metachromatic leukodystrophy (MLD) in England and Wales for eligible children. Children eligible are:
- children who have late infantile or early juvenile types, with no clinical signs or symptoms
- children who have the early juvenile type, with early clinical signs or symptoms, and who can still walk independently and have no cognitive decline.
NICE committee members concluded that “MLD is a rare, serious, and life-limiting condition that significantly affects the lives of people with the condition, their families and carers.” They commended the patient organisations for their submissions providing detailed feedback from a survey on the effect of Libmeldy® on quality of life. They also acknowledged that Libmeldy®, manufactured by Orchard Therapeutics, is an innovative technology and represents a step-change in managing MLD.
Vivienne Clark, Chair of The MLD Support Association UK, said: “We are delighted with today’s announcement from NICE confirming that Libmeldy® has been recommended for use in the treatment of MLD for pre-symptomatic Late infantile or Early Juvenile and early-symptomatic Early Juvenile children. This is a major development in our fight to eradicate MLD and we would like to wholeheartedly thank all the stakeholders, clinicians and researchers for their continued hard work and dedication in making this treatment a reality. We would also like to acknowledge all the affected children and their families whose lives have been affected by this condition – many of whom selflessly supported our research despite no possibility of benefitting from this treatment – their courage, bravery and strength has played the greatest role in this achievement.”
Georgina Morton, CEO of ArchAngel MLD Trust and patient expert, said: “We would like to thank NHS England and NICE for recognising the immeasurable suffering of MLD patients and the life-changing potential of Libmeldy®. This recommendation is a salute to the tireless commitment of many clinicians, advocates, investors and affected families and heralds a pivotal move towards vanquishing this appalling disease. It also facilitates an important step closer to newborn screening for MLD and a future which allows all eligible patients to access this remarkable treatment at the earliest opportunity.”
Nicola Elson, a patient expert, said: “We are completely overjoyed with the decision from NICE to recommend Libmeldy® for use in England and Wales for eligible children. With an MLD diagnosis for two of our children, one untreated and the other seven years post-transplant, we have witnessed first-hand the pain, torture and devastation this monstrous condition unleashes on innocent children. We have also seen the results of this ground-breaking treatment and the second chance at life it brings. We are thrilled those other children will have access to this therapy.”
Orchard Therapeutics CEO, Professor Bobby Gaspar, M.D., PhD said: “Today’s landmark decision by NHS England follows a thoughtful and comprehensive value assessment by NICE and represents a major milestone for the MLD community, Orchard, and the entire field of HSC gene therapy”.
Bob Stevens, Group Chief Executive of The MPS Society, said: “The decision by NICE to recommend Libmeldy® marks a milestone in the evolution of treatments and therapies for rare metabolic conditions. It is the first time that gene therapy has been recommended in England for any of these diseases and what was once science fiction has now become science fact. Today as a result of collaboration, we have another treatment option to add to the existing innovative therapies for one of our diseases, MLD. Today, hope grew a little larger for our community.”
The final evaluation document (FED) can be found here.
When NICE recommends a treatment the NHS must make sure it is available within 3 months.
We would like to thank all the MLD community, clinicians and patient groups for all their submissions, representation at committee meetings and information shared through the surveys and personal stories.
– END –